Yu Chen1, Li Xing1, William J. Welsh1,
Weida Tong2, Roger Perkins2, and
Daniel Sheehan3
A QSAR Study of the Binding of Suspected Endocrine Disrupting Compounds
(EDCs) to the Estrogen Receptor
1Department of Chemistry & Center for Molecular
Electronics, University of Missouri-St. Louis, St. Louis, MO 63121, USA.
2R.O.W. Sciences, Inc., Jefferson, AR, 72079, USA.
3National Center for Toxicological Research (NCTR), Jefferson,
AR 72079, USA.
The recent cloning of the estrogen receptor (ER) 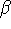 subtype suggests that the selective effects of certain estrogenic compounds
(e.g., tamoxifen) arises from control of different subsets of
estrogen-responsive promoters by the two ER subtypes,
subtype suggests that the selective effects of certain estrogenic compounds
(e.g., tamoxifen) arises from control of different subsets of
estrogen-responsive promoters by the two ER subtypes,
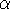 and
and 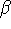 .
In order to identify the similarity/dissimilarity between these
two ER subtypes in terms of ligand recognition and binding,
separate 3D-QSAR models were developed for both the
.
In order to identify the similarity/dissimilarity between these
two ER subtypes in terms of ligand recognition and binding,
separate 3D-QSAR models were developed for both the
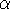 and
and 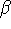 .
ER based on Relative Binding Affinity (RBA) data for a diverse
training set of 31 estrogenic compounds. Both 3D-QSAR models, which
correlated the observed RBA values for the training-set compounds
with their calculated 3D steric and electrostatic fields, exhibited
excellent self-consistency (r2 = 0.99) and predictive ability
(q2 > 0.65). Values of RBA predicted by these 3D-QSAR
models for a test set of compounds (i.e., not used to construct the
models) exhibited excellent agreement with experimental data.
Inspection of the corresponding CoMFA contour maps for
.
ER based on Relative Binding Affinity (RBA) data for a diverse
training set of 31 estrogenic compounds. Both 3D-QSAR models, which
correlated the observed RBA values for the training-set compounds
with their calculated 3D steric and electrostatic fields, exhibited
excellent self-consistency (r2 = 0.99) and predictive ability
(q2 > 0.65). Values of RBA predicted by these 3D-QSAR
models for a test set of compounds (i.e., not used to construct the
models) exhibited excellent agreement with experimental data.
Inspection of the corresponding CoMFA contour maps for
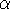 and
and 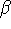 .
ER suggests subtle differences in their steric and electrostatic
requirements for ligand binding.
.
ER suggests subtle differences in their steric and electrostatic
requirements for ligand binding.
 Back to Program Page
Back to Program Page
 Back to Main Page
Back to Main Page
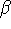 subtype suggests that the selective effects of certain estrogenic compounds
(e.g., tamoxifen) arises from control of different subsets of
estrogen-responsive promoters by the two ER subtypes,
subtype suggests that the selective effects of certain estrogenic compounds
(e.g., tamoxifen) arises from control of different subsets of
estrogen-responsive promoters by the two ER subtypes,
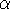 and
and 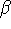 .
In order to identify the similarity/dissimilarity between these
two ER subtypes in terms of ligand recognition and binding,
separate 3D-QSAR models were developed for both the
.
In order to identify the similarity/dissimilarity between these
two ER subtypes in terms of ligand recognition and binding,
separate 3D-QSAR models were developed for both the
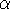 and
and 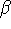 .
ER based on Relative Binding Affinity (RBA) data for a diverse
training set of 31 estrogenic compounds. Both 3D-QSAR models, which
correlated the observed RBA values for the training-set compounds
with their calculated 3D steric and electrostatic fields, exhibited
excellent self-consistency (r2 = 0.99) and predictive ability
(q2 > 0.65). Values of RBA predicted by these 3D-QSAR
models for a test set of compounds (i.e., not used to construct the
models) exhibited excellent agreement with experimental data.
Inspection of the corresponding CoMFA contour maps for
.
ER based on Relative Binding Affinity (RBA) data for a diverse
training set of 31 estrogenic compounds. Both 3D-QSAR models, which
correlated the observed RBA values for the training-set compounds
with their calculated 3D steric and electrostatic fields, exhibited
excellent self-consistency (r2 = 0.99) and predictive ability
(q2 > 0.65). Values of RBA predicted by these 3D-QSAR
models for a test set of compounds (i.e., not used to construct the
models) exhibited excellent agreement with experimental data.
Inspection of the corresponding CoMFA contour maps for
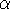 and
and 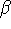 .
ER suggests subtle differences in their steric and electrostatic
requirements for ligand binding.
.
ER suggests subtle differences in their steric and electrostatic
requirements for ligand binding.